Speaker
Description
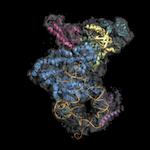
During protein synthesis at the ribosome numerous factors act early on the nascent polypeptide chain. These can be grouped into three major classes – chaperones that assist in folding, enzymes that modify the nascent chain and targeting factors that assist in protein localization. As all of them need access to the nascent polypeptide chain, they utilize partially overlapping binding sites at the ribosomal tunnel exit, but their interplay is poorly understood. Our data provide the structural framework for interactions of co-translational factors at the ribosomal tunnel exit. In yeast, the canonical Hsp70 protein Ssb acts together with the ribosome associated complex (RAC), which consists of the inactive Hsp70 protein Ssz and the Hsp40 protein Zuotin. Together, they form a unique chaperone triad at the ribosome. Structure determination of Ssb and RAC together with ribosome binding studies provide detailed insights into the interplay of this chaperone system, which evolved to link translation and protein folding.
References:
Weyer et al. (2017) NSMB 24:144-151.
Zhang et al. (2017) NSMB 24:611-19.
Gumiero et al. (2016) Nat. Comms. 7:13563.